Once upon a time, my girlfriend (and now wife) packed protein bars in her purse when we went out for the day.
They weren’t for her. They were for me.
She did it because she knew that if I didn’t eat protein every few hours, Mr. Hyde would come out.
I wouldn’t just get hungry. I would get angry. Hangry.
It was kind of pathetic, I know, but I thought that if you went for more than a few hours without protein, you’d lose muscle.
And when you think you’re losing muscle, you swear you can feel the your precious biceps disintegrating with every passing minute.
Well, I eventually canceled my magazine subscriptions and wised up.
You don’t have to eat protein every few hours to build muscle, and you could eat nothing for an entire day without losing any muscle to speak of.
That said, what’s optimal for gaining muscle?
Is there a difference between eating two and five servings of protein per day?
And if so, why?
Let’s find out, starting with a primer on the role protein plays in muscle growth.
Table of Contents
+
Want to listen to more stuff like this? Check out my podcast!
What Is Protein and Why Is It Important?
A protein is a compound that the body uses to create tissues, hormones, enzymes, and various other chemicals essential to life.
It’s made up of chains of smaller molecules known as amino acids, which are the basic building blocks of your body.
21 amino acids are needed to form proteins, and your body can produce 12 but must get the remaining 9 from the food you eat.
These 9 are known as essential amino acids and they are:
- Phenylalanine
- Valine
- Threonine
- Tryptophan
- Methionine
- Leucine
- Isoleucine
- Lysine
- Histidine
Many plants and animal tissues are rich in protein and serve as a food source, supplying these many amino acids that we need.
This is why you must eat protein: to provide your body with adequate essential amino acids.
Your body needs more or less protein based on various factors, including age and activity level.
Regular exercise, and weightlifting in particular, increases the body’s demand for protein, because it damages tissues that must be repaired.
Sedentary folk don’t need to eat as much protein as weightlifters, but they do need more than most people eat, and protein intake is more important than most people realize.
The reason for this is eating inadequate protein results in greater muscle loss as you get older, and the less lean mass you have in your later years, the more likely you are to die of all causes.
The bottom line is this: if you want to maintain your health as you age, you want to maintain your muscle.
And a high-protein diet (and resistance training) is vital to that.
Alright, now that we understand what protein is and why it matters, let’s move on to the next layer of this onion…
What Happens When You Eat Protein?
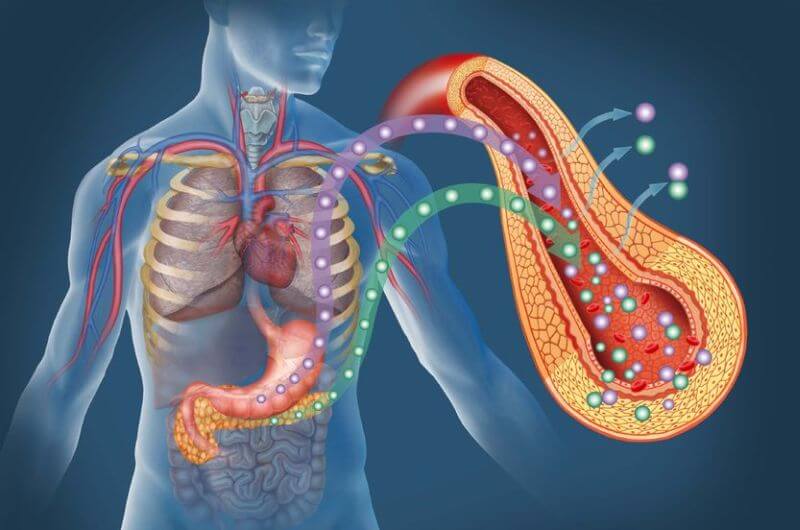
When you eat protein, acid and enzymes in your stomach break it down into amino acids.
Some forms of protein, like whey, break down quickly, whereas others, like egg, take quite a bit longer.
The amino acids make their way into the small intestine, which contains special cells that transport them into the blood. From there, they’re shuttled into cells everywhere in your body for use.
Now, many different things ultimately happen in your body when you eat protein, so let’s reframe the question to fit the context of this discussion:
How does eating protein affect your muscles?
And to understand that, we need to zero in on one of the essential amino acids in protein: leucine.
Leucine has a special place in bodybuilders’ hearts because it directly stimulates protein synthesis, which is the process by which amino acids are arranged into proteins that can then be used for muscle growth.
So…
- You eat a food containing protein, which is comprised of amino acids that are bound together.
- Your body breaks those bonds to obtain the free amino acids needed to build its own proteins.
- The presence of leucine tells the body that amino acids are available for use and to start building proteins.
- Your body complies, resulting in a pool of newly minted proteins that it can use to build and repair tissues, including muscle tissue.
As you can imagine, the amount of amino acids supplied by a meal affects the amount of muscle growth that can occur as a result.
This is why research shows that the leucine content of a meal directly affects the amount of protein synthesis that occurs as a result. In other words, high-leucine meals have a higher muscle-building potential than low-leucine meals.
And this is why it’s very important to consider the quality of the protein you’re eating.
What you want is a protein that is absorbed well by the body and rich in essential amino acids, and especially in leucine.
One of the reasons animal proteins like meat, eggs, and dairy are very popular among bodybuilders is they score very highly against those criteria.
(That isn’t to say you can’t build muscle effectively as a vegan, though. You can if you know what you’re doing.)
So, now that we have the basics of protein metabolism under our belts, we can tackle the subject at hand…
The Simple Science of Protein and Muscle Growth

All the cells in your body contain proteins that are constantly being broken down and built back up.
This applies to muscle tissue, of course, and these processes of breakdown and synthesis are simultaneously active at all times, but to varying degrees.
For example, when you’re in a fasted state, protein breakdown rates rise, and if they exceed synthesis rates, the result is muscle loss. This is called a state of negative protein balance.
When you eat protein, protein synthesis rise and once they exceed breakdown rates, the result is muscle gain. This is called a state of positive protein balance.
In this way, your body moves between anabolic and catabolic states each and every day.
Under normal health and dietary circumstances, muscle tissue is fairly stable and the cycle of cellular regeneration remains balanced.
This is why the average person doesn’t lose or gain muscle at an accelerated rate. On a day-to-day basis, there are no noticeable changes in total lean mass.
(That said, we do slowly lose lean mass as we age if we don’t take actions to stop it, but you get the point.)
Now, when we train our muscles we damage the cells in the muscle fibers, and this signals the body to increase protein synthesis rates to repair the damage.
Our bodies are smart, too, and want to adapt to better deal with the activity that caused the muscle damage. To do this, they add cells to the muscle fibers.
This is how muscles get bigger and stronger.
Thus, what we think of as just “muscle growth” is actually the result of protein synthesis rates exceeding protein breakdown rates over time.
In other words, when your body synthesizes (creates) more muscle proteins than it loses, you have gained muscle.
When it creates fewer than it loses, you have lost muscle.
And when it creates more or less the same number as it lost, you have neither gained nor lost muscle.
This is why bodybuilders do everything they can to elevate protein synthesis rates and suppress protein breakdown rates, including…
- High-protein and high-carb dieting
- Progressively overloading their muscles in the gym
- Ensuring they’re not in a calorie deficit
- Pre-workout and post-workout nutrition
- Eating protein before bed
- Limiting cardio
- Supplementation
- (And in many cases) steroids and other drugs
The goal of all of this is simply to keep protein synthesis rates as high above protein breakdown rates as possible for as many hours of the day as possible.
And as you can see, there are many factors in play that cumulatively determine whether you’re gaining or losing muscle.
Some are more important than others, too.
Eating protein before bed, for example, isn’t nearly as important as eating enough protein every day.
And that brings us to the central question of this article:
How important is protein timing? Does how frequently you eat protein influence the balance between protein synthesis and degradation (protein turnover) enough to matter?
Does Protein Timing Really Matter?

As I mentioned earlier, I used to believe that it absolutely did.
I was certain eating protein 4 to 6 times per day was vital for gaining muscle.
Well, it’s definitely not vital.
We can find conclusive evidence of this in research on the intermittent fasting style of dieting.
IF involves fasting (no food) for extended periods, followed by anywhere from 2 to 8-hour “feeding windows” and it’s well established that it doesn’t result in muscle loss.
For example, one study found that eating the entire day’s worth of protein in a 4-hour window (followed by 20 hours of fasting) didn’t result in muscle loss. Similar results have been seen in several other studies as well.
The bottom line is your muscle doesn’t wither if you miss a meal or fail to provide a constant supply of essential amino acids.
So long as you eat enough protein every day, you won’t lose muscle.
That said, there’s evidence that eating protein just 1 to 3 times per day isn’t optimal for building muscle.
First, let’s look at a study conducted by researchers at RMIT University. In it, 24 healthy, young men did a workout and then ate protein in one of several ways:
- 4 servings of 20 grams of protein, with 3 hours in between each.
- 2 servings of 40 grams of protein, with 6 hours in between each.
- 8 servings of 10 grams of protein, with 1.5 hours in between each.
And the result?
Muscle protein synthesis was significantly higher in group 1 than groups 2 and 3.
A study conducted by scientists at the University of Texas is also worth mentioning.
It found that protein synthesis was about 23% higher in people that ate three large meals containing 23 grams of protein plus three smaller meals containing 15 grams of essential amino acids compared to people that ate just three large meals alone.
Similar effects have been seen in athletes in a calorie deficit as well.
These findings aren’t surprising when you consider some of the things we know about how protein absorption affects protein metabolism.
Namely…
There’s a limit to the amount of protein that your body can digest, process, and then use for protein synthesis.
Research shows that this number is about 6 to 7 grams per hour for the average person (and it’s probably slightly higher in people with above-average muscularity).
There’s a limit to how high protein synthesis rates rise from a single dose of protein.
Scientists call this ceiling the “muscle full effect”, and once it has been reached, amino acids are no longer used for muscle building but are targeted for elimination instead (oxidation).
For example, in one study, researchers had young men eat varying amounts of egg protein after a workout and then measured protein synthesis rates.
They identified 20 grams of protein as the ceiling, as it resulted in 89% of the protein synthesis response conferred by 40 grams.
A similar study using whey protein found the same–20 grams was almost equally effective at elevating protein synthesis rates as 40 grams.
Similar effects were seen yet again in a study that found no statistically significant difference in protein synthesis rates after the ingestion of 30 and 90 grams of ground beef.
There’s a limit to how long protein synthesis rates remain elevated when you eat protein.
Research shows that muscle protein synthesis rates remain elevated for no longer than 3 hours regardless of how long amino acids remain in your bloodstream.
In other words, a large amount of protein may take, let’s say, 6 to 7 hours to fully digest and process, but protein synthesis rates will remain elevated for just 3 of those hours.
So if the body can only process about 7 grams of protein per hour for muscle protein synthesis…
…and if ~30 grams of protein maximally stimulates muscle protein synthesis…
…and if muscle protein synthesis lasts for no longer than 3 hours…
…then we can see why eating ~30 grams of protein every 3 to 4 hours results in more muscle protein accumulation over time than eating fewer, larger servings separated by longer periods.
By eating protein more frequently…
- You to keep protein synthesis rates above baseline for as many hours out of the day as possible.
- You allow for the amino acids provided to be utilized primarily for protein synthesis with very little earmarked for oxidation.
In other words, you to get the absolute most muscle growth out of each meal and your diet as a whole as possible.
The Bottom Line on Protein Timing

The evidence is clear: protein timing matters.
You’ll probably gain muscle faster eating 4 to 6 servings of protein every day than fewer.
That said, it’s not a deal maker or breaker like total protein intake and progressive overload.
If you prefer eating fewer, larger meals (a la intermittent fasting), you can still build plenty of muscle. It’s just not the optimal way of going about it.
What’s your take on protein timing? Have anything else to share? Let me know in the comments below!
Scientific References +
- Norton Layne, & Wilson Gabriel J. (n.d.). (PDF) Optimal protein intake to maximize muscle protein synthesis Examinations of optimal meal protein intake and frequency for athletes. Retrieved September 23, 2020, from https://www.researchgate.net/publication/288150322_Optimal_protein_intake_to_maximize_muscle_protein_synthesis_Examinations_of_optimal_meal_protein_intake_and_frequency_for_athletes
- Symons, T. B., Sheffield-Moore, M., Wolfe, R. R., & Paddon-Jones, D. (2009). A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. Journal of the American Dietetic Association, 109(9), 1582–1586. https://doi.org/10.1016/j.jada.2009.06.369
- Witard, O. C., Jackman, S. R., Breen, L., Smith, K., Selby, A., & Tipton, K. D. (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. American Journal of Clinical Nutrition, 99(1), 86–95. https://doi.org/10.3945/ajcn.112.055517
- Moore, D. R., Robinson, M. J., Fry, J. L., Tang, J. E., Glover, E. I., Wilkinson, S. B., Prior, T., Tarnopolsky, M. A., & Phillips, S. M. (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. American Journal of Clinical Nutrition, 89(1), 161–168. https://doi.org/10.3945/ajcn.2008.26401
- Atherton, P. J., Etheridge, T., Watt, P. W., Wilkinson, D., Selby, A., Rankin, D., Smith, K., & Rennie, M. J. (2010). Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. American Journal of Clinical Nutrition, 92(5), 1080–1088. https://doi.org/10.3945/ajcn.2010.29819
- Phillips, S. M., & van Loon, L. J. C. (2011). Dietary protein for athletes: From requirements to optimum adaptation. Journal of Sports Sciences, 29(SUPPL. 1). https://doi.org/10.1080/02640414.2011.619204
- Paddon-Jones, D., Sheffield-Moore, M., Aarsland, A., Wolfe, R. R., & Ferrando, A. A. (2005). Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. American Journal of Physiology - Endocrinology and Metabolism, 288(4 51-4). https://doi.org/10.1152/ajpendo.00291.2004
- Areta, J. L., Burke, L. M., Ross, M. L., Camera, D. M., West, D. W. D., Broad, E. M., Jeacocke, N. A., Moore, D. R., Stellingwerff, T., Phillips, S. M., Hawley, J. A., & Coffey, V. G. (2013). Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. Journal of Physiology, 591(9), 2319–2331. https://doi.org/10.1113/jphysiol.2012.244897
- Varady, K. A. (2011). Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obesity Reviews, 12(7). https://doi.org/10.1111/j.1467-789X.2011.00873.x
- Stote, K. S., Baer, D. J., Spears, K., Paul, D. R., Harris, G. K., Rumpler, W. V., Strycula, P., Najjar, S. S., Ferrucci, L., Ingram, D. K., Longo, D. L., & Mattson, M. P. (2007). A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. American Journal of Clinical Nutrition, 85(4), 981–988. https://doi.org/10.1093/ajcn/85.4.981
- Keogh, J. B., Pedersen, E., Petersen, K. S., & Clifton, P. M. (2014). Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clinical Obesity, 4(3), 150–156. https://doi.org/10.1111/cob.12052
- Soeters, M. R., Lammers, N. M., Dubbelhuis, P. F., Ackermans, M., Jonkers-Schuitema, C. F., Fliers, E., Sauerwein, H. P., Aerts, J. M., & Serlie, M. J. (2009). Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. The American Journal of Clinical Nutrition, 90(5), 1244–1251. https://doi.org/10.3945/ajcn.2008.27327
- Rasmussen, B. B., & Phillips, S. M. (2003). Contractile and nutritional regulation of human muscle growth. In Exercise and Sport Sciences Reviews (Vol. 31, Issue 3, pp. 127–131). Lippincott Williams and Wilkins. https://doi.org/10.1097/00003677-200307000-00005
- Bautmans, I., Van Puyvelde, K., & Mets, T. (2009). Sarcopenia and functional decline: Pathophysiology, prevention and therapy. In Acta Clinica Belgica (Vol. 64, Issue 4, pp. 303–316). Acta Clinica Belgica. https://doi.org/10.1179/acb.2009.048
- Chargé, S. B. P., & Rudnicki, M. A. (2004). Cellular and Molecular Regulation of Muscle Regeneration. In Physiological Reviews (Vol. 84, Issue 1, pp. 209–238). Physiol Rev. https://doi.org/10.1152/physrev.00019.2003
- Biolo, G., Tipton, K. D., Klein, S., & Wolfe, R. R. (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. American Journal of Physiology - Endocrinology and Metabolism, 273(1 36-1). https://doi.org/10.1152/ajpendo.1997.273.1.e122
- Biolo, G., Maggi, S. P., Williams, B. D., Tipton, K. D., & Wolfe, R. R. (1995). Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology - Endocrinology and Metabolism, 268(3 31-3). https://doi.org/10.1152/ajpendo.1995.268.3.e514
- Norton, L. E., Layman, D. K., Bunpo, P., Anthony, T. G., Brana, D. V., & Garlick, P. J. (2009). The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. Journal of Nutrition, 139(6), 1103–1109. https://doi.org/10.3945/jn.108.103853
- Kimball, S. R., & Jefferson, L. S. (2006). Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. Journal of Nutrition, 136(1). https://doi.org/10.1093/jn/136.1.227s