Key Takeaways
- Autophagy is the process of breaking down parts of or entire cells into amino acids and fatty acids.
- Autophagy plays an important role in aging and protecting against disease and dysfunction.
- There’s very little evidence that fasting is an effective way to increase autophagy, that you can do anything to significantly increase autophagy above natural, healthy levels, or that doing so would even be desirable.
According to an ever-growing number of popular doctors, researchers, and influencers and “gurus,” autophagy is the next big health breakthrough.
By making simple changes to your diet and lifestyle, they say, you can harness the power of this mysterious physiological phenomenon and . . .
- “Cleanse” your body of harmful toxins
- Slow or even reverse aging
- Protect against various types of disease, including cancer, dementia, and heart disease
- Feel more revitalized and rejuvenated
In many cases, the arguments sound pretty convincing. Buzzwords, whiteboards, and diagrams abound.
In fact, if the strategies shared (usually fasting or low-carb dieting or a combination of both) only worked half as well as many “experts” claim, the long-term results could still be life-changing.
And so it’s no surprise that autophagy is one of the hottest “health hacks” around right now.
It really shouldn’t be, however, and you’ll learn why in this article. By the end, you’re going to understand . . .
- What autophagy is
- How it works
- How it impacts your health
- How it relates to fasting
- What you can and can’t (and should and shouldn’t) do to “optimize” it
- And more
Ready? Keep reading!
Want to listen to more stuff like this? Check out my podcast!
What Is Autophagy?
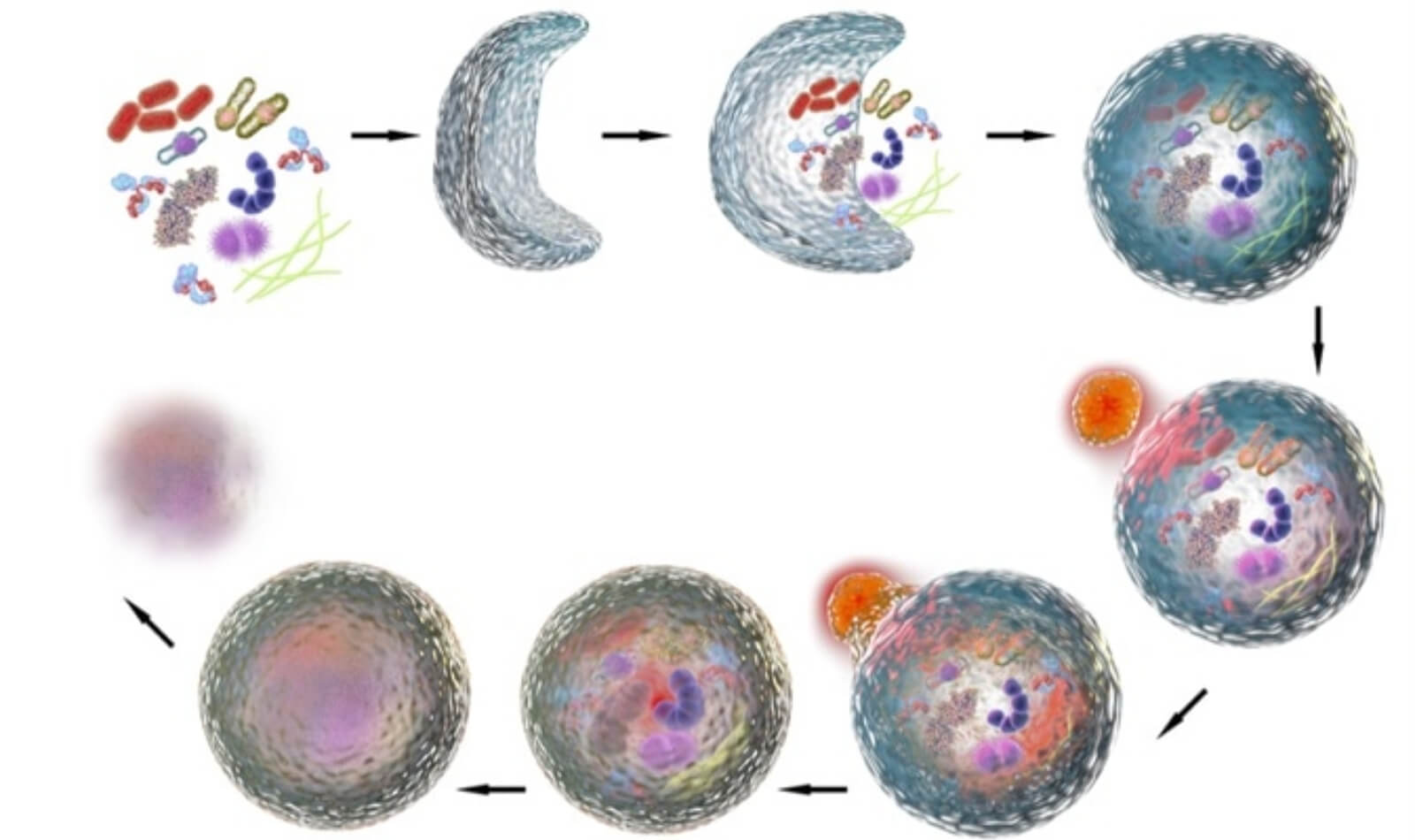
Autophagy is a cellular process whereby various waste products that have built up within cells or in some cases entire cells are recycled into nutrients that can then be used for growth, repair, and fuel.
The word comes from the Greek prefix “auto,” meaning “self,” and “phagy,” meaning “to eat.” In other words, “self-eating.”
Although autophagy has recently become a catchword in the health and fitness space, scientists have known about this process for decades. In fact, the term was coined by the french scientist Christian de Duve, who also discovered the lysosome (more on this in a minute), earning him a Nobel prize in 1974.
To understand how autophagy works and why it matters, you need to first understand a bit about how cells function.
In order to produce energy, small structures inside your cells called mitochondria consume nutrients and convert them into adenosine triphosphate (ATP). This chemical is the basic fuel that powers the myriad processes that keep your body alive.
In this way, mitochondria are the “power plants” of your cells, and like any power plants, they produce waste that must be disposed of. And in this case, it mostly consists of dead or damaged parts of cells and unused proteins.
The removal of this waste is important because if enough builds up in the cells, it can interfere with their ability to function properly.
To clean up these leftover byproducts, small structures called lysosomes patrol the insides of your cells, gobble up the trash, and break it down into amino acids and fatty acids. They also help prevent disease by attacking viruses and bacteria.
Think of lysosomes as cellular janitors and autophagy as how they go about their jobs.
Lysosomes do more than just “house cleaning” as well—if a cell is infected, cancerous, or damaged beyond repair, they can also destroy it and recycle its components.
So, that’s what autophagy is, now let’s learn a bit more about its effects in the body.
Autophagy and Your Health
Here’s one thing modern health gurus have right about autophagy: It’s not just healthy, it’s absolutely essential.
That’s why it’s always occurring in every cell in the body, and when it becomes compromised, you can develop many types of diseases and ailments.
For example, when scientists use drugs to turn off autophagy in mice, they can develop all kinds of health problems such as cancer, heart and liver dysfunction, retarded growth, anemia, and obesity.
Most of the studies on autophagy have been done on isolated cells (in-vitro) or animals, but at this point it’s generally accepted that autophagy plays an important role in warding off disease and dysfunction in humans as well.
That’s why many people claim that autophagy can . . .
- Slow or even reverse aging
- Protect against neurodegenerative diseases
- Reduce the risk of cancer and heart disease
Let’s take a closer look at each of these points.
Autophagy and Aging
The term “aging” refers to a progressive decline of physiological function.
This can manifest in many ways, including slowed recovery after exercise, reduced insulin sensitivity, decreased joint function, and others.
More importantly, aging also comes with an increased risk of many diseases from cancer to heart disease to dementia.
Older cells often contain a greater amount of cellular waste, and at least in mice, worms, and fruit flies, aging is also associated with a decline in autophagy.
Some people have taken this to mean that just about anything and everything associated with aging—from wrinkles to muscle weakness—can be cured if you can just increase autophagy.
That may or may not be true, but at this point we simply don’t know because the research isn’t available. The evidence confirms that autophagy affects aging in humans, but there’s currently no reliable way to control this process.
This whole “more/better autophagy = less/better aging” argument is reminiscent of the hype around telomeres from several years ago.
In case you missed it, scientists found that the lifespan of our cells is largely determined by finger-like structures on the end of our DNA called telomeres.
Every time a cell replicates, telomeres get a little bit shorter, and this gave rise to the theory that it might be possible to slow aging by limiting or reversing telomere shrinkage.
This kicked off a frenzy of research into telomeres starting in the mid-1970s. Nearly five decades later, we have a much better understanding of the function of telomeres, but we still don’t have a reliable way to measure their length, prevent them from shortening, or increase their length.
In other words, the science is currently about as useful as a picture of water to a person lost in a desert.
Autophagy is in the same boat.
Yes, it’s a natural biological process related to aging, and yes, we know that its decline is probably bad, but we don’t have a reliable way of using this knowledge to significantly impact aging.
Autophagy and Neurodegenerative Diseases
The term “neurodegenerative disease” includes any disease that involves the loss of structure or function of the nervous system, and particularly the brain.
Some of the most common examples include Parkinson’s, Huntington’s, and Alzheimer’s diseases, and multiple sclerosis.
Scientists are still teasing out the primary factors that cause and contribute to these diseases, and one potential candidate involves the buildup of “misfolded” proteins.
Every structure in the body is largely made of protein—from microscopic mitochondria to major muscle groups—and the construction of them requires extreme attention to detail.
Most of the time, things work more or less perfectly, resulting in incredibly complex structures that work exactly as intended. Sometimes, though, the body’s “protein machinery” makes mistakes, producing “misfolded proteins” that can’t function properly.
If too many misfolded proteins accumulate in the body, they can interfere with cellular function and eventually cause disease. For example, scientists believe that a buildup of misfolded proteins may be one of the main contributors to Alzheimer’s disease.
It’s not entirely clear why this buildup occurs and it’s even less clear how to treat or prevent it, but it’s possible that insufficient or impaired autophagy could be a factor.
Autophagy does involve rounding up and recycling misfolded proteins, so it stands to reason that the decrease in autophagy associated with aging would result in more misfolded proteins in our bodies. And it’s even possible that at some point in our lives, the accumulation of misfolded proteins could outpace our body’s ability to destroy them.
In this way, autophagy could help protect against neurodegenerative disease by cleaning up these misfolded proteins before they have a chance to cause problems.
That said, until we know more about this relationship and, more importantly, how to actively and safely regulate it, we can’t do anything special (beyond “healthy living”) to increase our neurological resilience.
Autophagy and Cancer
Cancer is a disease caused by uncontrolled division of abnormal cells in a part of the body.
If these defective, cancerous cells are allowed to multiply unchecked, they can develop into a mass called a tumor. Over time, this can interfere with many bodily functions and eventually cause death.
And guess what? All of us have had cancerous cells in our body, but in most cases, our bodies are able to identify and destroy them before the problems begin.
In many case, these cancerous cells are eliminated through the process of autophagy. This is why scientists think that autophagy can help protect against cancer in three ways:
- Keeping cells healthier and thus less likely to become cancerous in the first place
- Disassembling and recycling cancerous cells
- Reducing the effects of cancerous cells on nearby healthy cells
As proof of this, some animal and in-vitro research shows that when the genes that increase autophagy are destroyed, tumor growth increases.
A recent review of the research on autophagy and cancer conducted by scientists at Soonchunhyang University Seoul Hospital came to more or less the same conclusion: One of the primary ways your body destroys cancerous cells before they can replicate and spread is through autophagy.
This is all well and good, but unfortunately, as with aging and neurodegenerative disease, there’s still no reliable way for us to manipulate autophagy to become more protective against cancer.
Again, we can focus on healthy living through proper diet, nutrition, exercise, sleep hygiene, stress management, and the like, but beyond that, there currently isn’t any reliable way to boost or enhance autophagy.
Is Intermittent Fasting the Best Way to “Turn On” Autophagy?

These days, if you read, listen to, or watch anything on autophagy, you’ll also end up learning about fasting, and intermittent fasting in particular.
The reason for this is simple: blood markers of autophagy increase when the body’s in a fasted state (scientists currently can’t directly measure autophagy, only observe chemical changes associated with it).
The nitty gritty details of how this works are beyond the scope of this article, but suffice to say that when insulin and amino acid levels rise, autophagy drops. Conversely, when insulin and amino acid levels drop, autophagy rises.
For example, in one study conducted by scientists at The University of Texas, just 3.5 grams of leucine (which minimally impacts insulin levels) was enough to significantly decrease blood markers of autophagy for three hours in young men and women.
Why do insulin and amino acids impact autophagy? Scientists aren’t sure yet, but a few potential explanations are as follows:
- When energy is plentiful, as it is after eating a meal, your cells may have less of a need to recycle old amino acids for fuel (why eat leftovers when a fresh meal was just served?).
- The increase in autophagy during periods of fasting may be a side effect of other biological processes that also increase during fasting. For example, fasting also increases levels of AMPK, which is a protein that helps cells store and process nutrients and also increases autophagy.
- The body may use fasting as an opportunity to destroy old or damaged cells for fuel that would be left alone under normal conditions.
I mentioned above that there’s currently no way to directly measure autophagy in humans. Instead, we have to look at various chemicals in the blood (blood markers) that we believe, based on other research, are associated with autophagy.
The methods used for measuring these blood markers are still relatively new and unreliable, which means we shouldn’t be quick to draw hard-and-fast conclusions from them. In time, it’s very possible that newer, more effective research tools will come along and invalidate much of what is currently believed.
This is a subtle but important point because many phony pundits like to blur the lines between these “best guesses” at what’s going on in the body and valid, observable, meaningful improvements in health and function.
The bottom line, however, is that while the relationship between fasting and autophagy is certainly an interesting topic, there are still far more unknowns than knowns.
That hasn’t stopped Internet gurus from promoting intermittent fasting as the single best way to increase autophagy, and thus a powerful strategy for improving health, immunity, and longevity.
Their logic looks like this:
A = Intermittent fasting increases autophagy
B = Autophagy is essential for slowing aging and avoiding disease
C = Intermittent fasting is essential for slowing aging and avoiding disease
This is a fine hypothesis to be tested with rigorous scientific study, but rigorous scientists the fake YouTube doctors (chiropractors and “naturopaths”) are not.
Instead, they’re often charlatans who present their information as gospel, even the “the ancient secret of health,” as Dr. Jason Fung, one of the main proponents of the fasting-for-autophagy fad, likes to say.
To “prove” the wonders of intermittent fasting, these dubious guys and gals often point to studies like this one, conducted by scientists at Keio University Graduate School of Pharmaceutical Sciences.
If you take such research at face value (i.e., simply read the abstracts), you might conclude that just one or two days of fasting here and there can breathe new life into virtually every organ in your body. In fact, this is what many people openly claim online.
When you dig into the details, though, you learn otherwise.
For instance, in the study cited above, the scientists took eight mice and gave half of them unlimited access to food and starved the others for two days. Then, all mice were euthanized and blood and tissue samples collected to measure markers of autophagy.
And sure enough, the mice that ate nothing showed significantly greater levels of blood markers of autophagy. Hooray for fasting!
Or not.
There are a few rather obvious problems with this study:
- It was performed with just eight mice over two days, which is not a reliable model for long-term changes in human health.
- The starved mice lost 17% of their body weight by the end of day one and 23% by the end of day two, a massive drop in body weight. Would humans need to lose this much weight to see a significant increase in autophagy? Would the benefits disappear once you resume your normal diet? Could you get the same benefits by losing weight over a longer period of time? No studies, including this one, provide any answers.
- The results are based on blood markers of autophagy, which as mentioned previously aren’t entirely reliable.
That’s why this study, and others like it, are interesting fodder for discussion and further research, but most definitely not proof that intermittent fasting is an effective, scientifically proven way to live longer and healthier.
And especially when you consider that there’s scant human research on autophagy in general and very little understood about how it truly affects aging in humans.
In fact, it’s not clear that increasing autophagy above normal levels would be a good thing even if we could accomplish it. While autophagy does involve “cleaning” cells of waste products, it also often entails destroying cells, which isn’t necessarily something you want to accelerate.
In other words, while amphetamines would certainly help janitors do more work, maybe the outcome wouldn’t be a cleaner building.
An illustrative example of how little we really know about autophagy and general health comes from a study conducted by scientists at Ben-Gurion University of the Negev.
Researchers found higher levels of blood markers associated with cellular autophagy in obese people than normal people, and that larger and more insulin resistant fat cells showed higher levels of autophagy than smaller and less insulin resistant ones.
In other words, autophagy appeared to be higher in less healthy people and cells than healthy ones, which flies directly into the teeth of claims that higher levels of autophagy are always good or always a good sign.
The scientists weren’t sure why this was the case, either. Maybe it’s because the body senses an excess of nutrients and is revving up autophagy to cope with the influx of proteins. Or, as the authors posited, maybe “ . . . it is also possible that increased autophagy signifies a process underlying increased cell death of hypertrophied adipocytes [swollen fat cells].”
That is, the scientists may have been witnessing the body using autophagy to kill off cells that were being pushed to their (literal) breaking points due to overeating. A sort of cellular triage, if you will.
This theory is supported by another study conducted by scientists at the University of Campinas, which also found that obesity led to runaway autophagy in the fat cells of both mice and humans.
So instead of thinking about increasing autophagy with “biohacks” or strategies like intermittent fasting, it’s better to think about not doing things that might decrease autophagy below healthy levels.
A good analogy for this is the cooling system in an engine.
Most engines have an optimal operating temperature. Things must be just right—not too hot or too cold—for the ideal function of all of the parts.
Likewise, humans require a certain amount of autophagy for optimal cellular function, and the body carefully and automatically increases and decreases autophagy as needed.
Now, if the cooling system of an engine completely shuts down, it quickly overheats and stops working. And by the same token, cooling the engine as much as possible isn’t good, either.
Similarly, increasing autophagy as much as possible (however you might do that, if you can at all), isn’t necessarily better than generally living healthily and letting our body set the pace.
Ironically, given what we currently know, it’s entirely possible that eating well and staying active and fit are enough to get all of the benefits of autophagy, with no need for intermittent fasting.
For example, studies conducted by scientists at the University of Virginia, University of Texas, and University of Padova have shown that exercise can increase the expression of genes that are responsible for autophagy.
Studies conducted by scientists at the Biopotentials Sleep Center and University of Michigan Medical Center have also found that autophagy is linked to sleep cycles, so it’s possible that maintaining good sleep hygiene could go a long way in supporting healthy levels of autophagy.
It’s hard to sell pills, powders, and PDFs when that’s your position, though. And especially when you’re up against quacks who promise metabolic transformation at the flick of a dietary or lifestyle switch.
So, to summarize:
It’s not clear that intermittent fasting is an effective way to increase autophagy or that taking special measures to increase autophagy is even desirable.
The Bottom Line on Autophagy

Autophagy is a cellular process whereby various waste products or entire cells are recycled into nutrients that can then be used for growth, repair, and fuel.
It’s essential for proper bodily function, and when impaired, can contribute to the development of types of various disease and dysfunction.
In humans, it’s often claimed that enhancing autophagy can:
- Slow or even reverse aging
- Protect against neurodegenerative diseases
- Reduce the risk of cancer and heart disease
It’s very likely that autophagy plays a key role in all of these processes, but it’s not clear that it can be increased through special measures or that doing so would even be desirable for health and longevity.
Some studies show that fasting can increase blood markers of autophagy temporarily, which has sparked a veritable fasting frenzy among experts and laymen alike.
When you dive into the research, though, you quickly realize that it’s often wildly misrepresented and exaggerated as a ploy to sell supplements, books, courses, and the like. These self-styled gurus also conveniently overlook research that shows simple steps like staying active may support autophagy more than fancy diet protocols ever could.
So the bottom line is this:
Yes, autophagy is an important bodily function for living a long, healthy, vibrant life . . . just like breathing, converting glucose into ATP, digesting food, and many other natural functions that your body carries out every second of every day without you even realizing it.
That doesn’t mean, however, that it’s easy or even desirable to tamper with autophagy through dietary modifications, supplements, or other “special” methods.
Instead, focus on eating well, staying active and fit, and sleeping well, and you’ll have the best chances of your body rewarding you with healthy, vitality, and longevity.
What’s your take on autophagy? Have anything else to share? Let me know in the comments below!
Scientific References +
- Ma, D., Li, S., Molusky, M. M., & Lin, J. D. (2012). Circadian autophagy rhythm: A link between clock and metabolism? In Trends in Endocrinology and Metabolism (Vol. 23, Issue 7, pp. 319–325). NIH Public Access. https://doi.org/10.1016/j.tem.2012.03.004
- He, Y., Cornelissen-Guillaume, G. G., He, J., Kastin, A. J., Harrison, L. M., & Pan, W. (2016). Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiology International, 33(5), 553–560. https://doi.org/10.3109/07420528.2015.1137581
- Grumati, P., Coletto, L., Schiavinato, A., Castagnaro, S., Bertaggia, E., Sandri, M., & Bonaldo, P. (2011). Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy, 7(12), 1415–1423. https://doi.org/10.4161/auto.7.12.17877
- He, C., Sumpter, R., & Levine, B. (2012). Exercise induces autophagy in peripheral tissues and in the brain. Autophagy, 8(10), 1548–1551. https://doi.org/10.4161/auto.21327
- Lira, V. A., Okutsu, M., Zhang, M., Greene, N. P., Laker, R. C., Breen, D. S., Hoehn, K. L., & Yan, Z. (2013). Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB Journal, 27(10), 4184–4193. https://doi.org/10.1096/fj.13-228486
- Nuñez, C. E., Rodrigues, V. S., Gomes, F. S., De Moura, R. F., Victorio, S. C., Bombassaro, B., Chaim, E. A., Pareja, J. C., Geloneze, B., Velloso, L. A., & Araujo, E. P. (2013). Defective regulation of adipose tissue autophagy in obesity. International Journal of Obesity, 37(11), 1473–1480. https://doi.org/10.1038/ijo.2013.27
- Kovsan, J., Blüher, M., Tarnovscki, T., Klöting, N., Kirshtein, B., Madar, L., Shai, I., Golan, R., Harman-Boehm, I., Schön, M. R., Greenberg, A. S., Elazar, Z., Bashan, N., & Rudich, A. (2011). Altered autophagy in human adipose tissues in obesity. Journal of Clinical Endocrinology and Metabolism, 96(2). https://doi.org/10.1210/jc.2010-1681
- Yamamoto, J., Kamata, S., Miura, A., Nagata, T., Kainuma, R., & Ishii, I. (2015). Differential adaptive responses to 1- or 2-day fasting in various mouse tissues revealed by quantitative PCR analysis. FEBS Open Bio, 5, 357–368. https://doi.org/10.1016/j.fob.2015.04.012
- Gottlieb, R. A., Andres, A. M., Sin, J., & Taylor, D. P. J. (2015). Untangling Autophagy Measurements. Circulation Research, 116(3), 504–514. https://doi.org/10.1161/CIRCRESAHA.116.303787
- Yoshii, S. R., & Mizushima, N. (2017). Monitoring and measuring autophagy. In International Journal of Molecular Sciences (Vol. 18, Issue 9). MDPI AG. https://doi.org/10.3390/ijms18091865
- Kim, J., Kundu, M., Viollet, B., & Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology, 13(2), 132–141. https://doi.org/10.1038/ncb2152
- Bujak, A. L., Crane, J. D., Lally, J. S., Ford, R. J., Kang, S. J., Rebalka, I. A., Green, A. E., Kemp, B. E., Hawke, T. J., Schertzer, J. D., & Steinberg, G. R. (2015). AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metabolism, 21(6), 883–890. https://doi.org/10.1016/j.cmet.2015.05.016
- Glynn, E. L., Fry, C. S., Drummond, M. J., Timmerman, K. L., Dhanani, S., Volpi, E., & Rasmussen, B. B. (2010). Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. Journal of Nutrition, 140(11), 1970–1976. https://doi.org/10.3945/jn.110.127647
- Ribeiro, M., López de Figueroa, P., Blanco, F. J., Mendes, A. F., & Caramés, B. (2016). Insulin decreases autophagy and leads to cartilage degradation. Osteoarthritis and Cartilage, 24(4), 731–739. https://doi.org/10.1016/j.joca.2015.10.017
- Longo, V. D., & Mattson, M. P. (2014). Fasting: Molecular mechanisms and clinical applications. In Cell Metabolism (Vol. 19, Issue 2, pp. 181–192). NIH Public Access. https://doi.org/10.1016/j.cmet.2013.12.008
- Yun, C. W., & Lee, S. H. (2018). The roles of autophagy in cancer. In International Journal of Molecular Sciences (Vol. 19, Issue 11). MDPI AG. https://doi.org/10.3390/ijms19113466
- Mathew, R., Karantza-Wadsworth, V., & White, E. (2007). Role of autophagy in cancer. In Nature Reviews Cancer (Vol. 7, Issue 12, pp. 961–967). Nat Rev Cancer. https://doi.org/10.1038/nrc2254
- Rubinsztein, D. C., Gestwicki, J. E., Murphy, L. O., & Klionsky, D. J. (2007). Potential therapeutic applications of autophagy. In Nature Reviews Drug Discovery (Vol. 6, Issue 4, pp. 304–312). Nat Rev Drug Discov. https://doi.org/10.1038/nrd2272
- Ashraf, G., Greig, N., Khan, T., Hassan, I., Tabrez, S., Shakil, S., Sheikh, I., Zaidi, S., Akram, M., Jabir, N., Firoz, C., Naeem, A., Alhazza, I., Damanhouri, G., & Kamal, M. (2014). Protein Misfolding and Aggregation in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS & Neurological Disorders - Drug Targets, 13(7), 1280–1293. https://doi.org/10.2174/1871527313666140917095514
- Shamsi, T. N., Athar, T., Parveen, R., & Fatima, S. (2017). A review on protein misfolding, aggregation and strategies to prevent related ailments. In International Journal of Biological Macromolecules (Vol. 105, Issue Pt 1, pp. 993–1000). Elsevier B.V. https://doi.org/10.1016/j.ijbiomac.2017.07.116
- Gitler, A. D., Dhillon, P., & Shorter, J. (2017). Neurodegenerative disease: Models, mechanisms, and a new hope. In DMM Disease Models and Mechanisms (Vol. 10, Issue 5, pp. 499–502). Company of Biologists Ltd. https://doi.org/10.1242/dmm.030205
- Gotlib, I. H., Lemoult, J., Colich, N. L., Foland-Ross, L. C., Hallmayer, J., Joormann, J., Lin, J., & Wolkowitz, O. M. (2015). Telomere length and cortisol reactivity in children of depressed mothers. Molecular Psychiatry, 20(5), 615–620. https://doi.org/10.1038/mp.2014.119
- Starkweather, A. R., Alhaeeri, A. A., Montpetit, A., Brumelle, J., Filler, K., Montpetit, M., Mohanraj, L., Lyon, D. E., & Jackson-Cook, C. K. (2014). An integrative review of factors associated with telomere length and implications for biobehavioral research. In Nursing Research (Vol. 63, Issue 1, pp. 36–50). NIH Public Access. https://doi.org/10.1097/NNR.0000000000000009
- Shammas, M. A. (2011). Telomeres, lifestyle, cancer, and aging. Current Opinion in Clinical Nutrition and Metabolic Care, 14(1), 28–34. https://doi.org/10.1097/MCO.0b013e32834121b1
- Corey, D. R. (2009). Telomeres and Telomerase: From Discovery to Clinical Trials. In Chemistry and Biology (Vol. 16, Issue 12, pp. 1219–1223). Elsevier Ltd. https://doi.org/10.1016/j.chembiol.2009.12.001
- Martinez-Lopez, N., Athonvarangkul, D., & Singh, R. (2015). Autophagy and aging. Advances in Experimental Medicine and Biology, 847, 73–87. https://doi.org/10.1007/978-1-4939-2404-2_3
- Martinez-Vicente, M., & Cuervo, A. M. (2007). Autophagy and neurodegeneration: when the cleaning crew goes on strike. In Lancet Neurology (Vol. 6, Issue 4, pp. 352–361). Lancet Neurol. https://doi.org/10.1016/S1474-4422(07)70076-5
- Levine, B., & Kroemer, G. (2008). Autophagy in the Pathogenesis of Disease. In Cell (Vol. 132, Issue 1, pp. 27–42). NIH Public Access. https://doi.org/10.1016/j.cell.2007.12.018
- Niccoli, T., & Partridge, L. (2012). Ageing as a risk factor for disease. In Current Biology (Vol. 22, Issue 17, pp. R741–R752). Cell Press. https://doi.org/10.1016/j.cub.2012.07.024
- Kuma, A., Komatsu, M., & Mizushima, N. (2017). Autophagy-monitoring and autophagy-deficient mice. In Autophagy (Vol. 13, Issue 10, pp. 1619–1628). Taylor and Francis Inc. https://doi.org/10.1080/15548627.2017.1343770
- Casanova, J. E. (2017). Bacterial Autophagy: Offense and Defense at the Host–Pathogen Interface. In CMGH (Vol. 4, Issue 2, pp. 237–243). Elsevier Inc. https://doi.org/10.1016/j.jcmgh.2017.05.002
- Chiramel, A., Brady, N., & Bartenschlager, R. (2013). Divergent Roles of Autophagy in Virus Infection. Cells, 2(1), 83–104. https://doi.org/10.3390/cells2010083
- Jiang, P., & Mizushima, N. (2014). Autophagy and human diseases. In Cell Research (Vol. 24, Issue 1, pp. 69–79). Nature Publishing Group. https://doi.org/10.1038/cr.2013.161
- Ohsumi, Y. (2014). Historical landmarks of autophagy research. In Cell Research (Vol. 24, Issue 1, pp. 9–23). Nature Publishing Group. https://doi.org/10.1038/cr.2013.169
- Glick, D., Barth, S., & Macleod, K. F. (2010). Autophagy: Cellular and molecular mechanisms. In Journal of Pathology (Vol. 221, Issue 1, pp. 3–12). NIH Public Access. https://doi.org/10.1002/path.2697