Key Takeaways
- Muscle protein synthesis is the process your body uses to repair, grow, and strengthen muscle fibers.
- If you increase muscle protein synthesis and decrease muscle protein breakdown, then you build muscle.
- The best ways to increase muscle protein synthesis are to do lots of heavy strength training, eat enough calories and protein, and time your meals properly.
Building muscle can seem like a confusing process.
Some people say you need to use high reps in your workouts, others say you should just focus on getting strong.
Some say you need to eat a high-protein diet, others say it doesn’t matter.
Some say you need to eat a meal immediately before and/or after your workout, others say you just have to eat enough calories throughout the day.
How are you supposed to know who’s right?
Well, one way to sift through this muddle of facts, opinions, and pap is to zero in on exactly what you’re going for.
And if your goal is to build muscle, then you need to understand that all of these strategies are really aimed at one thing: muscle protein synthesis.
What’s that, you wonder?
Well, that’s what you’re going to learn in this article.
You’re going to learn what muscle protein synthesis is, why it’s so important for building muscle, the six best ways to increase muscle protein synthesis, and how to avoid the things that decrease muscle protein synthesis.
Let’s get started.
- What Is Muscle Protein Synthesis?
- How Protein Synthesis Affects Muscle Growth
- 6 Ways to Increase Muscle Protein Synthesis
- The Bottom Line on Muscle Protein Synthesis
Table of Contents
+Want to listen to more stuff like this? Check out my podcast!
What Is Muscle Protein Synthesis?
To synthesize something means to combine (a number of things) into a coherent whole.
When it comes to muscle protein synthesis, this means the creation of new muscle tissue from amino acids.
(If you want to learn what protein synthesis looks like at a cellular level, these two videos are a good place to start.)
Amino acids are small molecules that combine to form proteins, and they’re constantly being disassembled and reassembled in your body.
When the amino acids in muscle tissue are broken down, this is referred to as muscle protein breakdown.
These processes of breakdown and synthesis are simultaneously active at all times, but to varying degrees.
For example, when you’re in a fasted state, protein breakdown rates rise, and if they exceed synthesis rates, the result is muscle loss. This is called a state of negative protein balance.
When you eat protein, protein synthesis rates rise and once they exceed breakdown rates, the result is muscle gain. This is called a state of positive protein balance.
In this way, your body moves between anabolic and catabolic states each and every day.
Under normal health and dietary circumstances, muscle tissue is fairly stable and the cycle of cellular regeneration remains balanced.
Here’s what it looks like throughout the day:
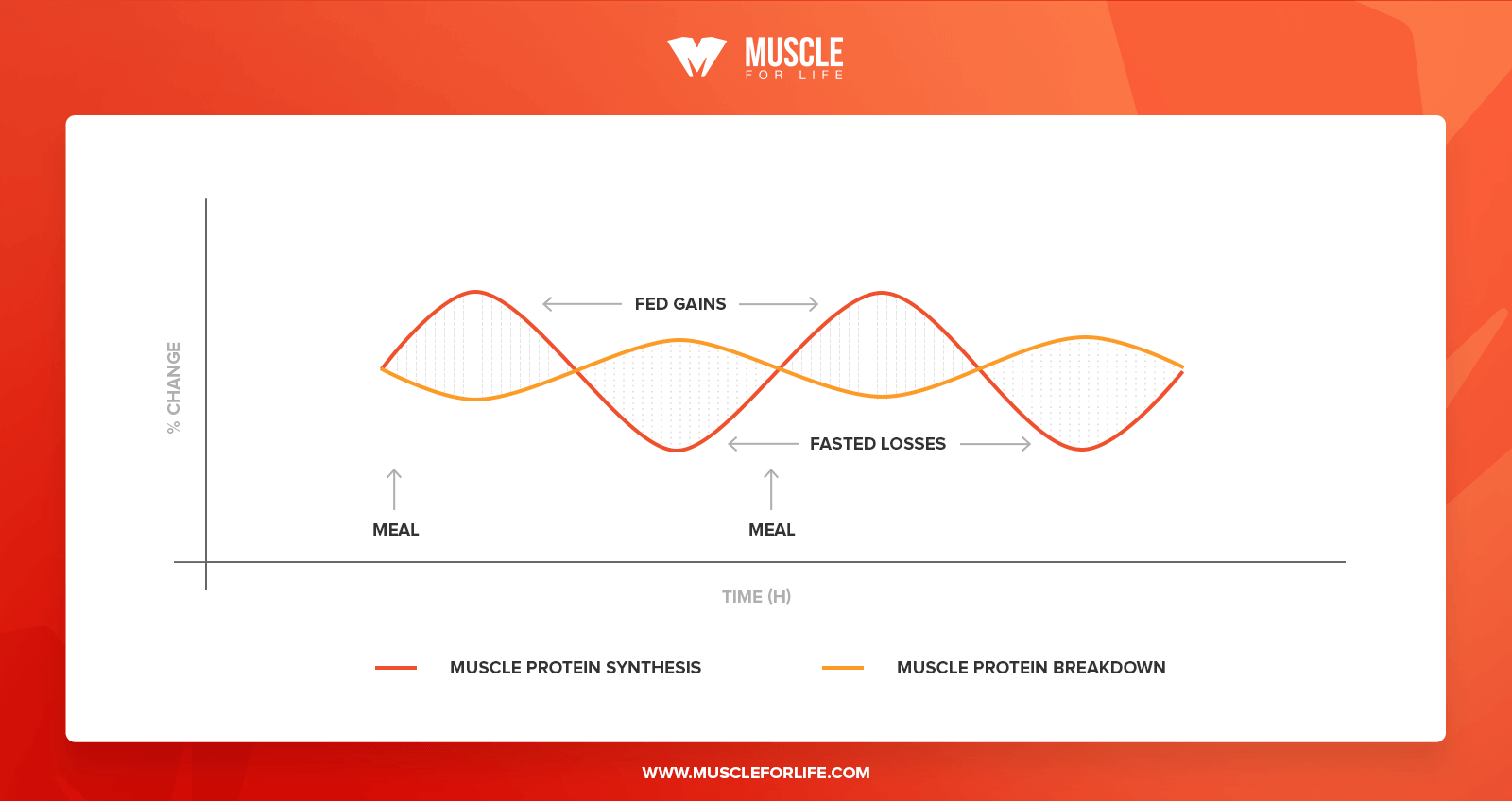
As you can see, every rise in protein synthesis is matched by a subsequent rise in protein breakdown, and they more or less balance each other out.
This is why the average person doesn’t lose or gain muscle over time. On a day-to-day basis, there are no noticeable changes in total lean mass.
(That said, we do slowly lose lean mass as we age if we don’t take actions to stop it, but you get the point.)
How Protein Synthesis Affects Muscle Growth
So, how are we supposed to gain muscle if protein synthesis and protein breakdown cancel each other out?
By nudging protein synthesis slightly higher than protein breakdown over time.
When protein synthesis rates outpace protein breakdown rates for weeks and months, our muscles grow larger and stronger.
Thus, what we think of as “muscle growth” is actually the result of protein synthesis rates exceeding protein breakdown rates over time.
In other words, when your body synthesizes (creates) more muscle proteins than it loses, you have gained muscle.
When it creates fewer than it loses, you have lost muscle.
And when it creates more or less the same number as it lost, you have neither gained nor lost muscle.
This is why bodybuilders do everything they can to elevate protein synthesis rates and suppress protein breakdown rates, including…
- High-protein and high-carb dieting
- Progressively overloading their muscles in the gym
- Ensuring they’re not in a calorie deficit
- Pre-workout and post-workout nutrition
- Eating protein before bed
- Limiting cardio
- Supplementation
- (And in many cases) steroids and other drugs
The goal of all of this is simply to keep protein synthesis rates as high above protein breakdown rates as possible for as many hours of the day as possible.
And as you can see, there are many factors in play that cumulatively determine whether you’re gaining or losing muscle.
Now, something else worth mentioning is the kind of muscle protein synthesis that we’re aiming for. All kinds of exercise can increase muscle protein synthesis, including endurance sports like cycling, swimming, running, skiing, and hiking.
So, why aren’t these people jacked?
Well, three reasons:
- The protein synthesis is occurring mostly in the mitochondria, which are the little “power plants” of the cell. These mitochondria multiply and become more efficient, which makes you better at endurance sports, but they don’t increase muscle size. Strength training, on the other hand, increases protein synthesis in the actual muscle fibers, causing them to grow larger.
- There’s also a large rise in protein breakdown during endurance sports which cancels out the rise in protein synthesis.
- Most people who engage in these sports also restrict their calorie intake, which increases muscle protein breakdown rates.
So, what can you do to keep protein synthesis elevated long enough to build muscle?
A lot, actually.
6 Ways to Increase Muscle Protein Synthesis
If you want to build muscle, you have two goals:
- Increase muscle protein synthesis for as long as possible throughout the day.
- Reduce muscle protein breakdown for as long as possible throughout the day.
Let’s tackle the first part of this equation—increasing muscle protein synthesis.
Strength Training and Muscle Protein Synthesis
Since ancient times, we’ve known that lifting heavy things and putting them down builds muscle.
Just look at Socrates, who was also an avid wrestler:

When we train our muscles we damage the cells in the muscle fibers, and this signals the body to increase protein synthesis rates to repair the damage.
Here’s what this looks like throughout the day:
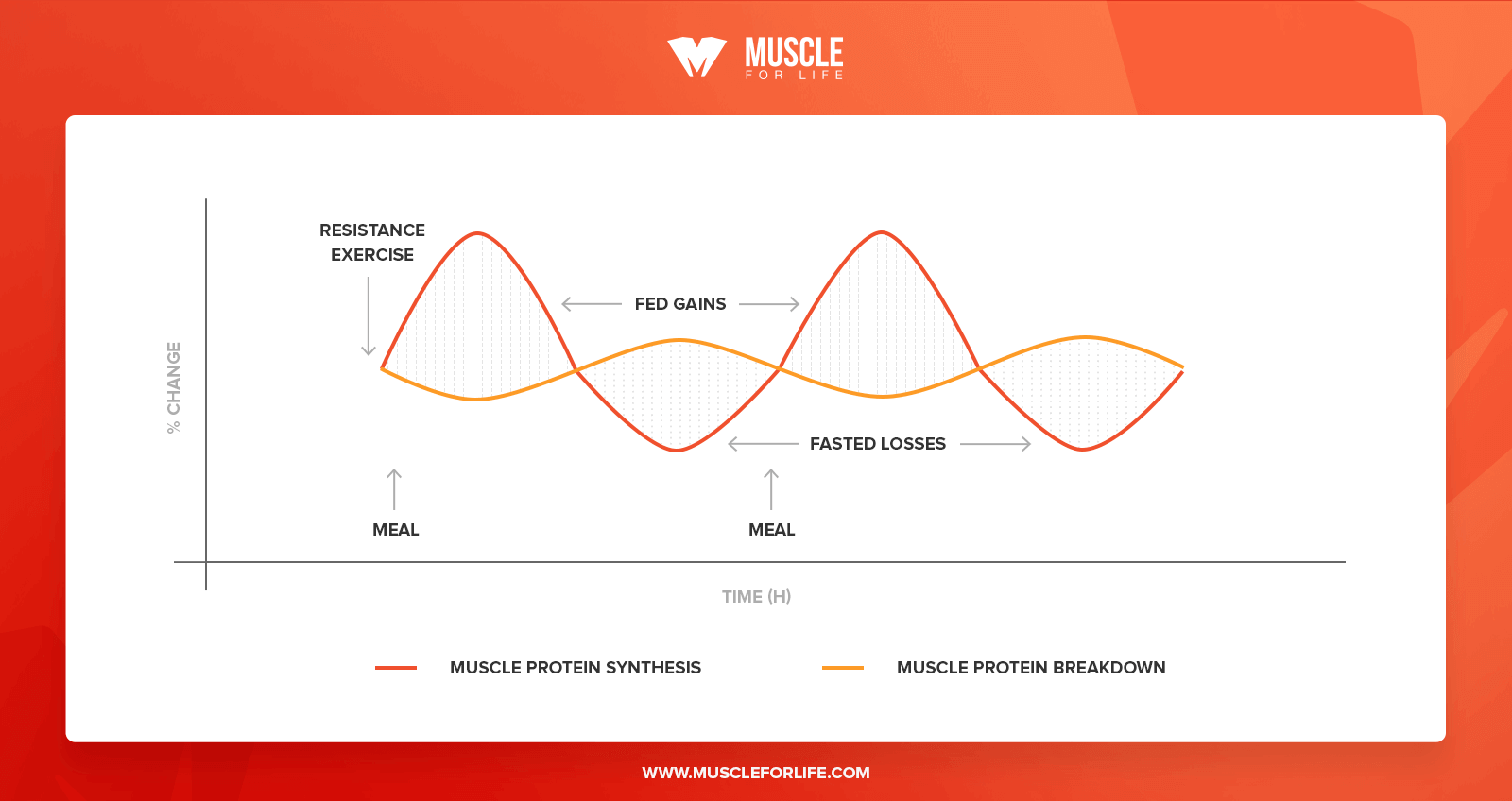
There are a few other things that occur inside the muscle cells when we train that also flip the switch on muscle protein synthesis, but the main one is mechanical tension. That is, pushing heavier and heavier weights over time.
After a workout, there’s a rapid and prolonged rise in muscle protein synthesis. This lasts anywhere from 3 days in newbies to less than 24 hours in advanced athletes. This is because your body becomes better at recovering from exercise, and thus doesn’t need to keep protein synthesis elevated for as long.
This becomes more important as you inch closer to your genetic potential, because your body becomes more and more resistant to the effects of strength training. It takes more sets to cause the same increase in muscle protein synthesis, and the increase drops back to baseline much faster.
This is why you have to increase your training volume over time to keep muscle protein synthesis elevated and thus, your muscle building machinery humming along at full bore.
That’s all well and good, but there’s also a sharp rise in muscle protein breakdown that happens when you lift weights.
In fact, after a workout the rate of muscle protein breakdown is significantly higher than the rate of muscle protein synthesis. After an hour or two in the gym, you’re in a more catabolic (muscle wasting) state.
In other words, strength training increases protein synthesis and protein breakdown.
Now, muscle protein breakdown rates do drop after a time, and strength training still has a net muscle-building effect over the long-term. But, as a natural weightlifter, you want to do everything you can to reduce the muscle protein breakdown caused by lifting.
That’s where nutrition becomes important, which we’ll discuss next.
Calories and Muscle Protein Synthesis
Yes, you need to eat enough protein, which isn’t news to anyone trying to build muscle, but what many people don’t know is you need to eat enough calories as well.
You see, your body burns a certain amount of energy every day, which can be measured in calories (one calorie is “the amount of energy required to heat 1 kg of water 1 degree Celsius at one atmosphere of pressure”).
This is known as your “total daily energy expenditure,” or TDEE.
Your body gets the energy it needs to stay alive from food, of course, and the relationship between how much energy you eat and burn is known as energy balance, and it greatly impacts both your body weight and muscle growth.
Namely, if you feed your body less energy than it burns, you’ve created an energy (or calorie) deficit that will result in weight loss if sustained for a period of time.
It will also impair your body’s ability to create muscle proteins, which slows down (or even halts) muscle growth.
The physiology in play is fairly complex, but the long story short is when you restrict your body’s energy intake, it shifts to an “energy conservation” mode wherein certain bodily functions are given priority over others.
Building bigger muscles isn’t vital for survival and requires quite a bit of energy, so it’s rather low on the list.
Furthermore, a calorie deficit can reduce anabolic and increase catabolic hormone levels, causing a systemic shift away from muscle protein synthesis and toward muscle protein breakdown.
All this is why it’s commonly believed that you can’t build muscle and lose fat at the same time (which isn’t exactly accurate, which we’ll talk more about soon).
This is also why women can lose their periods while restricting calories for fat loss. When in an energy-deprived state, their bodies can neglect the non-vital and energy-intensive process of menstruation.
So, when we want to build muscle as quickly as possible, what do we have to ensure regarding our calorie intake?
You got it—we have to ensure we’re not in a calorie deficit, and this is true regardless of our dietary protocol.
Regardless of the dietary protocol we follow–intermittent fasting, carb cycling, flexible dieting, or whatever else—if we’re a calorie deficit more often than not, we’re going to have a hard time keeping muscle protein synthesis rates high enough to build muscle.
If you want to maximize muscle protein synthesis, make sure you eat enough calories to maintain or gain body weight. For most people, this will be about 14 to 16 calories per pound of bodyweight.
Protein and Muscle Protein Synthesis

In order for protein synthesis to occur, you need two things:
- The stimulus
- The raw materials
Typically, people think of strength training as the stimulus for muscle growth and protein as the raw material, but protein plays both roles.
As you probably know, you need to eat enough protein so that your body has the amino acids it needs to build new muscle protein.
The actual amount of protein you need for this, though, is minimal. Your body is very efficient at recycling proteins, and you don’t need to eat that much to make sure you have the right amount for rebuilding muscle tissue.
Protein has another trick up its sleeve, though: When you eat protein, it directly stimulates muscle protein synthesis, much like strength training.
This effect is so powerful that even people who don’t lift weights can gain muscle when they increase their protein intake.
This has been proven in study after study after study. The main reason for this seems to be thanks to the amino acid leucine, which is particularly abundant in animal proteins like whey, and also some plant proteins, like pea.
Now, you may have heard that your body can only absorb 20 grams of protein at a time. This idea came from the fact that, although eating protein increases protein synthesis, eating more than 20 to 25 grams doesn’t seem to cause a much larger rise in protein synthesis.
Other, more recent studies, though, have shown that larger amounts of protein could have a meaningful benefit over smaller doses.
Not only do 40-gram doses of protein cause a larger rise in muscle protein synthesis, they also do a better job of tamping down muscle protein breakdown.
Eating protein also reduces muscle protein breakdown, which, as you’ll recall, is the second piece of the puzzle when it comes to building muscle.
This is one of the reasons it’s often recommended that you eat protein in the hours before and/or after your workouts. Not only does this further stimulate protein synthesis above what you could get from the workout, it also decreases the muscle protein breakdown that occurs as a result of weightlifting.
So, taken as a whole, you can see how eating more protein can improve our body composition:
- It provides the raw materials our muscles need to repair, adapt, and grow.
- It directly stimulates muscle protein synthesis, much like strength training.
- It reduces muscle protein breakdown, which normally occurs after strength training.
How much protein does it take to get the job done?
To maximize protein synthesis, eat at least 0.8 to 1.2 grams of protein per pound of body weight per day.
Protein Timing and Muscle Protein Synthesis

Ask any somewhat serious gym rat what you should do with your diet, and chances are good that this will be their answer:
Make sure you eat protein after your workouts.
Some might also tell you to eat protein before your workouts, during your workouts, and before bed.
Is any of that necessary, though?
Well, yes and no.
The first thing you need to know about muscle protein synthesis and meal timing is that your total protein intake throughout the day is far, far, farrrrr more important than when you eat that protein throughout the day.
When it comes to keeping your muscle protein synthesis levels elevated, the total amount of protein you eat per day is your first, second, and third priority.
Think of it this way:
If you eat the right amount of protein (0.8 to 1.2 grams per pound), but cram it all in a single meal, you’ll still probably get better results than someone who eats a suboptimal amount of protein (say, 0.5 grams per pound) that’s perfectly spaced throughout the day.
Of course, there’s no reason you need to choose between those two extremes.
Assuming you are eating enough total protein throughout the day, paying some attention to when you eat that protein can help you build more muscle.
First, let’s look at a study conducted by researchers at RMIT University in Australia. The researchers put 24 healthy, young men through a strength training workout and then fed them protein in one of several ways:
- 4 servings of 20 grams of protein, with 3 hours in between each.
- 2 servings of 40 grams of protein, with 6 hours in between each.
- 8 servings of 10 grams of protein, with 1.5 hours in between each.
And the result?
Muscle protein synthesis was significantly higher in group 1 than groups 2 and 3.
A study conducted by scientists at the University of Texas is also worth mentioning.
It found that protein synthesis was about 23% higher in people that ate three large meals containing 23 grams of protein plus three smaller meals containing 15 grams of essential amino acids compared to people that ate just three large meals alone.
Similar effects have been seen in athletes in a calorie deficit as well.
These findings aren’t surprising when you consider some of the things we know about how protein absorption affects protein metabolism.
Namely…
There’s a limit to the amount of protein that your body can digest, process, and then use for protein synthesis. Research shows that this number is about 6 to 7 grams per hour for the average person (and it’s probably slightly higher in people with above-average muscularity).
There’s a limit to how high protein synthesis rates rise from a single dose of protein.
Scientists call this ceiling the “muscle full effect”, and once it has been reached, amino acids are no longer used for muscle building but are targeted for elimination instead (oxidation).
For example, in one study, researchers had young men eat varying amounts of egg protein after a workout and then measured protein synthesis rates.
They identified 20 grams of protein as the ceiling, as it resulted in 89% of the protein synthesis response conferred by 40 grams.
A similar study using whey protein found the same 20-gram dose was almost equally effective at elevating protein synthesis rates as 40 grams.
Similar effects were seen yet again in a study that found no statistically significant difference in protein synthesis rates after the ingestion of 30 and 90 grams of ground beef.
There’s a limit to how long protein synthesis rates remain elevated when you eat protein.
Research shows that muscle protein synthesis rates remain elevated for no longer than 3 hours regardless of how long amino acids remain in your bloodstream.
In other words, a large amount of protein may take, let’s say, 6 to 7 hours to fully digest and process, but protein synthesis rates will remain elevated for just 3 of those hours.
So if the body can only process about 7 grams of protein per hour for muscle protein synthesis…
…and if ~30 grams of protein maximally stimulates muscle protein synthesis…
…and if muscle protein synthesis lasts for no longer than 3 hours…
…then we can see why eating ~30 grams of protein every 3 to 4 hours results in more muscle protein accumulation over time than eating fewer, larger servings separated by longer periods.
And if you look at the research, this is exactly what you see.
A study published in the Journal of the International Society of Sports Nutrition looked at all of the relevant data surrounding this topic, and concluded that to reap all of the muscle-building benefits of protein, you’ll probably do best abiding by this simple rule:
Eat 0.2 to 0.25 grams of protein per pound of body weight spread across at least 4 meals throughout the day (or slightly more, if you prefer).
Another review published by the same researchers recommended that you place two of those meals at least an hour or so before and after your workout.
Assuming you needed 200 grams of protein per day, you’d want your meal schedule to look something like this:
7 AM
40 grams of protein
8 AM
Lift weights
9 AM
40 grams of protein
12 PM
40 grams of protein
6 PM
40 grams of protein
9 PM
40 grams of protein
Or this, for afternoon training:
8 AM
40 grams of protein
12 PM
40 grams of protein
3 PM
40 grams of protein
5 PM
Lift weights
6 PM
40 grams of protein
9 PM
40 grams of protein
The bottom line is that protein timing can help you increase muscle protein synthesis and thus muscle growth, but implementing this strategy is much easier than most people would have you believe.
To reap the muscle-building benefits of protein timing, eat 0.2 to 0.25 grams of protein per pound of body weight spread across at least 4 meals throughout the day (or slightly more, if you prefer), and sandwich your workouts between two of the meals.
BCAAs and Muscle Protein Synthesis

If you’ve read anything about protein, muscle growth, and supplements, then you’ve heard of BCAAs.
In case you’ve been living in an abandoned missile silo, though, let’s get you up to speed.
Branched-chain amino acids, or BCAAs for short, are a group of three essential amino acids (amino acids that your body must get from your diet):
- Leucine
- Isoleucine
- Valine
Leucine is the star of the trio, as it directly stimulates protein synthesis via the activation of an enzyme responsible for cell growth known as the mammalian target of rapamycin, or mTOR.
Isoleucine is number two on the list, as it improves glucose metabolism and increases glucose uptake in the muscles.
Valine is a distant third as it doesn’t seem to do much of anything when compared to leucine and isoleucine.
You find high amounts of these amino acids in quality proteins such as meat, eggs and dairy products, with whey protein isolate being particularly high.
There’s no question that taking BCAAs increases muscle protein synthesis, which is why you’ll see just about every fitness guru on the Internet hawking one brand or another.
But, there are two very important points about BCAAs that these people aren’t telling you:
1. Research commonly cited that demonstrates muscle-related benefits of BCAA supplementation was done with subjects that didn’t eat enough protein.
For example, this study is one of the poster boys for selling BCAAs. It examined the effects of BCAA supplementation on a group of wrestlers in a calorie deficit. After three weeks, the supplement group, who ingested an additional 52 grams of BCAAs per day preserved more muscle and lost a bit more fat than the control group (who didn’t supplement at all).
Sounds pretty cool, right? Well, what you won’t hear is that subjects, whose average weight was about 150 pounds, were eating a paltry ~80 grams of protein per day. If we look at research on the protein needs of athletes in a calorie deficit, we learn that they should have been eating double that amount of protein to preserve lean mass.
So all that study really tells us is if we feel like eating half the amount of protein we should be eating, a BCAA supplement can help mitigate the damage. Not too exciting.
Other studies that demonstrate various muscle-related benefits of BCAA supplementation have promising abstracts, but are almost always hampered by lack of dietary control and/or low protein intake, and in almost all cases, subjects are training fasted, which is a very important point we’ll talk more about in a minute.
2. You can simply get your BCAAs from food instead, and this is cheaper and far more satisfying.
Research that demonstrates the anabolic effects of BCAA supplementation before, during, and after exercise is often used to sell the powders. But this misses the forest for the trees.
What such research tells us is that acutely raising BCAA levels (and leucine in particular) before and after exercise helps us build more muscle.
You don’t need to use BCAA or leucine supplements to do this, though.
In fact, there’s research to the contrary: food, and whey protein specifically, may be even more effective than amino acid drinks.
This is why I recommend you eat 30 to 40 grams of protein before and after working out, and why I use whey protein for these meals. It’s cheaper than BCAA powders, tastes better, and is more effective.
The one proven use of BCAAs is to help prevent muscle protein breakdown during fasted training. There’s also some evidence they may reduce muscle damage during really long endurance events, too. You can learn more about that here.
Although BCAAs can get the job done, a better choice is a fancy-sounding molecule called β-Hydroxy β-Methylbutyrate (HMB).
HMB is a substance formed when your body metabolizes leucine.
HMB is often sold as a muscle-building aid but the research purported to demonstrate these benefits is shaky at best, hindered most by design flaws. Thus, I’m not comfortable making any claims about muscle growth.
There is one benefit of HMB that’s well established, however: it’s an extremely effective anti-catabolic agent.
That is, it’s very good at preventing muscle protein breakdown, which means you’ll recover faster from your workouts and experience less muscle soreness (and the free acid form shows the most promise in this regard).
It also has no effect whatsoever on insulin levels, which means it won’t break your fasted state like food.
These things make HMB perfect for use with fasted training.
Its powerful anti-catabolic effects and non-existent insulin effects means you reap all the fat loss benefits of training fasted without any of the problems relating to muscle loss or insulin secretion.
It’s also worth noting that HMB is superior to leucine in suppressing muscle breakdown because it’s more anti-catabolic than its “parent” amino acid.
If you’re interested in learning more about the benefits of HMB, check out this article.
The bottom line is that BCAAs do increase muscle protein synthesis, but unless you’re eating a really low protein diet or training fasted, there’s no reason you can’t get enough BCAAs from your diet.
Sleep and Muscle Protein Synthesis

In 2014, the Center for Disease Control declared that insufficient sleep is a public health epidemic.
According to polling conducted by the National Sleep Foundation, 43 percent of Americans between the ages of 13 and 64 say they rarely or never get a good night’s sleep on weeknights. Sixty percent say that they experience a sleep problem every night or almost every night.
Thus, it’s not surprising that sleep loss is also linked with lower rates of muscle growth and higher levels of body fat.
Researchers from the University of Chicago illustrated this nicely in a 2010 study.
They split people into two groups:
- Group 1 slept 8.5 hours per night, on the high end of what most experts recommend.
- Group 2 slept 5.5 hours per night, which is normal for many Americans.
Then, both groups were put on a 1,500 calorie per day diet for two weeks.
At the end of the experiment, the group that slept 5.5 hours per night lost 60% more muscle and 55% less fat than the group that got enough shuteye.
The researchers didn’t test why this was the case in this study, but other research hints at the cause. It’s well known that sleep loss decreases anabolic hormones like testosterone, growth hormone, and IGF-1, which play a key role in stimulating muscle protein synthesis and reducing muscle protein breakdown.
Sleep loss also hurts athletic performance (especially strength) and recovery which is another way that it can indirectly interfere with your ability to build muscle.
Studies on rats also show that sleep deprivation alone can cause muscle wasting.
If you want to learn more about how sleep impacts your performance, body composition, and health, and how to get more quality sleep, check out this article.
The bottom line is that if you want to do everything you can to increase muscle protein synthesis, decrease muscle protein breakdown, and improve your body composition, you’ll make sleep just as much of a priority as your diet and training plan.
The Bottom Line on Muscle Protein Synthesis
Building muscle can be confusing, but at bottom, you have a simple goal:
Keep muscle protein synthesis elevated above muscle protein breakdown. Do that, and you’ll have no trouble building muscle.
Elevating muscle protein synthesis comes down to providing the proper stimulus in the form of strength training, eating enough protein throughout the day, and timing your protein intake appropriately, and making sure you get enough sleep.
More specifically, you want to…
- Do lots of heavy, compound strength training.
- Eat at least 0.8 to 1.2 grams of protein per pound of body weight.
- Eat 0.2 to 0.25 grams of protein per pound of body weight spread out over at least 4 meals throughout the day, with your workout in between two of those meals.
- Take BCAAs or HMB if you’re training fasted.
- Get at least 7 to 9 hours of sleep per night (ideally 8 to 9).
Do that, and you’ll have no trouble keeping your protein synthesis rates high and making gains.
What’s your take on muscle protein synthesis? Have anything else to share? Let me know in the comments below!
Scientific References +
- Mônico-Neto, M., Antunes, H. K. M., Dattilo, M., Medeiros, A., Souza, H. S., Lee, K. S., De Melo, C. M., Tufik, S., & De Mello, M. T. (2013). Resistance exercise: A non-pharmacological strategy to minimize or reverse sleep deprivation-induced muscle atrophy. Medical Hypotheses, 80(6), 701–705. https://doi.org/10.1016/j.mehy.2013.02.013
- Dattilo, M., Antunes, H. K. M., Medeiros, A., Mônico Neto, M., Souza, H. S., Tufik, S., & De Mello, M. T. (2011). Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Medical Hypotheses, 77(2), 220–222. https://doi.org/10.1016/j.mehy.2011.04.017
- Reilly, T., & Piercy, M. (1994). The effect of partial sleep deprivation on weight-lifting performance. Ergonomics, 37(1), 107–115. https://doi.org/10.1080/00140139408963628
- Fullagar, H. H. K., Skorski, S., Duffield, R., Hammes, D., Coutts, A. J., & Meyer, T. (2015). Sleep and Athletic Performance: The Effects of Sleep Loss on Exercise Performance, and Physiological and Cognitive Responses to Exercise. In Sports Medicine (Vol. 45, Issue 2, pp. 161–186). Springer International Publishing. https://doi.org/10.1007/s40279-014-0260-0
- Dattilo, M., Antunes, H. K. M., Medeiros, A., Mônico Neto, M., Souza, H. S., Tufik, S., & De Mello, M. T. (2011). Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Medical Hypotheses, 77(2), 220–222. https://doi.org/10.1016/j.mehy.2011.04.017
- Nedeltcheva, A. V., Kilkus, J. M., Imperial, J., Schoeller, D. A., & Penev, P. D. (2010). Insufficient sleep undermines dietary efforts to reduce adiposity. Annals of Internal Medicine, 153(7), 435–441. https://doi.org/10.7326/0003-4819-153-7-201010050-00006
- Wilkinson, D. J., Hossain, T., Hill, D. S., Phillips, B. E., Crossland, H., Williams, J., Loughna, P., Churchward-Venne, T. A., Breen, L., Phillips, S. M., Etheridge, T., Rathmacher, J. A., Smith, K., Szewczyk, N. J., & Atherton, P. J. (2013). Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. Journal of Physiology, 591(11), 2911–2923. https://doi.org/10.1113/jphysiol.2013.253203
- Wilson, J. M., Lowery, R. P., Joy, J. M., Walters, J. A., Baier, S. M., Fuller, J. C., Stout, J. R., Norton, L. E., Sikorski, E. M., Wilson, S. M. C., Duncan, N. M., Zanchi, N. E., & Rathmacher, J. (2013). β-Hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. British Journal of Nutrition, 110(3), 538–544. https://doi.org/10.1017/S0007114512005387
- Rowlands, D. S., & Thomson, J. S. (2009). Effects of β-Hydroxy-β-methylbutyrate supplementation during resistance training on strength,body composition, and muscle damage in trained and untrained young men: A meta-analysis. Journal of Strength and Conditioning Research, 23(3), 836–846. https://doi.org/10.1519/JSC.0b013e3181a00c80
- Kim, D.-H., Kim, S.-H., Jeong, W.-S., & Lee, H.-Y. (2013). Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. Journal of Exercise Nutrition and Biochemistry, 17(4), 169–180. https://doi.org/10.5717/jenb.2013.17.4.169
- Hulmi, J. J., Lockwood, C. M., & Stout, J. R. (2010). Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. In Nutrition and Metabolism (Vol. 7). Nutr Metab (Lond). https://doi.org/10.1186/1743-7075-7-51
- Mero, A. (1999). Leucine supplementation and intensive training. Sports Medicine, 27(6), 347–358. https://doi.org/10.2165/00007256-199927060-00001
- Sharp, C. P. M., & Pearson, D. R. (2010). Amino acid supplements and recovery from high-intensity resistance training. Journal of Strength and Conditioning Research, 24(4), 1125–1130. https://doi.org/10.1519/JSC.0b013e3181c7c655
- Yoshiharu Shimomura 1 , Yuko Yamamoto, Gustavo Bajotto, Juichi Sato, Taro Murakami, Noriko Shimomura, Hisamine Kobayashi, K. M. (n.d.). Nutraceutical effects of branched-chain amino acids on skeletal muscle - PubMed. Retrieved July 31, 2020, from https://pubmed.ncbi.nlm.nih.gov/16424141/
- Mourier, A., Bigard, A. X., De Kerviler, E., Roger, B., Legrand, H., & Guezennec, C. Y. (1997). Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. International Journal of Sports Medicine, 18(1), 47–55. https://doi.org/10.1055/s-2007-972594
- M A Staten, D M Bier, D. E. M. (n.d.). Regulation of valine metabolism in man: a stable isotope study - PubMed. Retrieved July 31, 2020, from https://pubmed.ncbi.nlm.nih.gov/6439027/
- Doi, M., Yamaoka, I., Fukunaga, T., & Nakayama, M. (2003). Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochemical and Biophysical Research Communications, 312(4), 1111–1117. https://doi.org/10.1016/j.bbrc.2003.11.039
- Anthony, J. C., Anthony, T. G., Kimball, S. R., & Jefferson, L. S. (2001). Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. Journal of Nutrition, 131(3). https://doi.org/10.1093/jn/131.3.856s
- Aragon, A. A., & Schoenfeld, B. J. (2013). Nutrient timing revisited: Is there a post-exercise anabolic window? Journal of the International Society of Sports Nutrition, 10(1), 5. https://doi.org/10.1186/1550-2783-10-5
- Schoenfeld, B. J., & Aragon, A. A. (2018). How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. In Journal of the International Society of Sports Nutrition (Vol. 15, Issue 1, p. 10). BioMed Central Ltd. https://doi.org/10.1186/s12970-018-0215-1
- Layne Norton. (n.d.). (PDF) Optimal protein intake to maximize muscle protein synthesis Examinations of optimal meal protein intake and frequency for athletes. Retrieved July 31, 2020, from https://www.researchgate.net/publication/288150322_Optimal_protein_intake_to_maximize_muscle_protein_synthesis_Examinations_of_optimal_meal_protein_intake_and_frequency_for_athletes
- Symons, T. B., Sheffield-Moore, M., Wolfe, R. R., & Paddon-Jones, D. (2009). A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. Journal of the American Dietetic Association, 109(9), 1582–1586. https://doi.org/10.1016/j.jada.2009.06.369
- https://pubmed.ncbi.nlm.nih.gov/19699838/
- Moore, D. R., Robinson, M. J., Fry, J. L., Tang, J. E., Glover, E. I., Wilkinson, S. B., Prior, T., Tarnopolsky, M. A., & Phillips, S. M. (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. American Journal of Clinical Nutrition, 89(1), 161–168. https://doi.org/10.3945/ajcn.2008.26401
- Atherton, P. J., Etheridge, T., Watt, P. W., Wilkinson, D., Selby, A., Rankin, D., Smith, K., & Rennie, M. J. (2010). Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. American Journal of Clinical Nutrition, 92(5), 1080–1088. https://doi.org/10.3945/ajcn.2010.29819
- Bilsborough, S., & Mann, N. (2006). A review of issues of dietary protein intake in humans. In International Journal of Sport Nutrition and Exercise Metabolism (Vol. 16, Issue 2, pp. 129–152). Human Kinetics Publishers Inc. https://doi.org/10.1123/ijsnem.16.2.129
- Phillips, S. M., & van Loon, L. J. C. (2011). Dietary protein for athletes: From requirements to optimum adaptation. Journal of Sports Sciences, 29(SUPPL. 1). https://doi.org/10.1080/02640414.2011.619204
- Paddon-Jones, D., Sheffield-Moore, M., Aarsland, A., Wolfe, R. R., & Ferrando, A. A. (2005). Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. American Journal of Physiology - Endocrinology and Metabolism, 288(4 51-4). https://doi.org/10.1152/ajpendo.00291.2004
- Areta, J. L., Burke, L. M., Ross, M. L., Camera, D. M., West, D. W. D., Broad, E. M., Jeacocke, N. A., Moore, D. R., Stellingwerff, T., Phillips, S. M., Hawley, J. A., & Coffey, V. G. (2013). Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. Journal of Physiology, 591(9), 2319–2331. https://doi.org/10.1113/jphysiol.2012.244897
- Schoenfeld, B. J., Aragon, A. A., & Krieger, J. W. (2013). The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. In Journal of the International Society of Sports Nutrition (Vol. 10, p. 53). BioMed Central. https://doi.org/10.1186/1550-2783-10-53
- Helms, E. R., Aragon, A. A., & Fitschen, P. J. (2014). Evidence-based recommendations for natural bodybuilding contest preparation: Nutrition and supplementation. In Journal of the International Society of Sports Nutrition (Vol. 11, Issue 1). BioMed Central Ltd. https://doi.org/10.1186/1550-2783-11-20
- Kim, I. Y., Schutzler, S., Schrader, A., Spencer, H. J., Azhar, G., Ferrando, A. A., & Wolfe, R. R. (2015). The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. American Journal of Physiology - Endocrinology and Metabolism, 310(1), E73–E80. https://doi.org/10.1152/ajpendo.00365.2015
- Macnaughton, L. S., Wardle, S. L., Witard, O. C., McGlory, C., Hamilton, D. L., Jeromson, S., Lawrence, C. E., Wallis, G. A., & Tipton, K. D. (2016). The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiological Reports, 4(15). https://doi.org/10.14814/phy2.12893
- Moore, D. R., Robinson, M. J., Fry, J. L., Tang, J. E., Glover, E. I., Wilkinson, S. B., Prior, T., Tarnopolsky, M. A., & Phillips, S. M. (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. American Journal of Clinical Nutrition, 89(1), 161–168. https://doi.org/10.3945/ajcn.2008.26401
- Trommelen, J., & van Loon, L. J. C. (2016). Pre-sleep protein ingestion to improve the skeletal muscle adaptive response to exercise training. In Nutrients (Vol. 8, Issue 12). MDPI AG. https://doi.org/10.3390/nu8120763
- Tipton, K. D., & Wolfe, R. R. (2001). Exercise, protein metabolism, and muscle growth. International Journal of Sport Nutrition, 11(1), 109–132. https://doi.org/10.1123/ijsnem.11.1.109
- Bray, G. A., Smith, S. R., De Jonge, L., Xie, H., Rood, J., Martin, C. K., Most, M., Brock, C., Mancuso, S., & Redman, L. M. (2012). Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: A randomized controlled trial. JAMA - Journal of the American Medical Association, 307(1), 47–55. https://doi.org/10.1001/jama.2011.1918
- Atherton, P. J., Etheridge, T., Watt, P. W., Wilkinson, D., Selby, A., Rankin, D., Smith, K., & Rennie, M. J. (2010). Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. American Journal of Clinical Nutrition, 92(5), 1080–1088. https://doi.org/10.3945/ajcn.2010.29819
- Macnaughton, L. S., Wardle, S. L., Witard, O. C., McGlory, C., Hamilton, D. L., Jeromson, S., Lawrence, C. E., Wallis, G. A., & Tipton, K. D. (2016). The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiological Reports, 4(15). https://doi.org/10.14814/phy2.12893
- Williams, N. I., Leidy, H. J., Hill, B. R., Lieberman, J. L., Legro, R. S., & De Souza, M. J. (2015). Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. American Journal of Physiology - Endocrinology and Metabolism, 308(1), E29–E39. https://doi.org/10.1152/ajpendo.00386.2013
- Tomiyama, A. J., Mann, T., Vinas, D., Hunger, J. M., Dejager, J., & Taylor, S. E. (2010). Low calorie dieting increases cortisol. Psychosomatic Medicine, 72(4), 357–364. https://doi.org/10.1097/PSY.0b013e3181d9523c
- Cangemi, R., Friedmann, A. J., Holloszy, J. O., & Fontana, L. (2010). Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell, 9(2), 236–242. https://doi.org/10.1111/j.1474-9726.2010.00553.x
- Pasiakos, S. M., Vislocky, L. M., Carbone, J. W., Altieri, N., Konopelski, K., Freake, H. C., Anderson, J. M., Ferrando, A. A., Wolfe, R. R., & Rodriguez, N. R. (2010). Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. Journal of Nutrition, 140(4), 745–751. https://doi.org/10.3945/jn.109.118372
- Zito, C. I., Qin, H., Blenis, J., & Bennett, A. M. (2007). SHP-2 regulates cell growth by controlling the mTOR/S6 kinase 1 pathway. Journal of Biological Chemistry, 282(10), 6946–6953. https://doi.org/10.1074/jbc.M608338200
- Robert R Wolfe. (n.d.). Skeletal muscle protein metabolism and resistance exercise - PubMed. Retrieved July 31, 2020, from https://pubmed.ncbi.nlm.nih.gov/16424140/
- Rasmussen, B. B., & Phillips, S. M. (2003). Contractile and nutritional regulation of human muscle growth. In Exercise and Sport Sciences Reviews (Vol. 31, Issue 3, pp. 127–131). Lippincott Williams and Wilkins. https://doi.org/10.1097/00003677-200307000-00005
- Schoenfeld, B. J., Ogborn, D., & Krieger, J. W. (2017). Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. Journal of Sports Sciences, 35(11), 1073–1082. https://doi.org/10.1080/02640414.2016.1210197
- MacDougall, J. D., Gibala, M. J., Tarnopolsky, M. A., MacDonald, J. R., Interisano, S. A., & Yarasheski, K. E. (1995). The time course for elevated muscle protein synthesis following heavy resistance exercise. Canadian Journal of Applied Physiology, 20(4), 480–486. https://doi.org/10.1139/h95-038
- Schoenfeld, B. J. (2010). The mechanisms of muscle hypertrophy and their application to resistance training. In Journal of Strength and Conditioning Research (Vol. 24, Issue 10, pp. 2857–2872). J Strength Cond Res. https://doi.org/10.1519/JSC.0b013e3181e840f3
- Rasmussen, B. B., & Phillips, S. M. (2003). Contractile and nutritional regulation of human muscle growth. In Exercise and Sport Sciences Reviews (Vol. 31, Issue 3, pp. 127–131). Lippincott Williams and Wilkins. https://doi.org/10.1097/00003677-200307000-00005
- Weinert, D. J. (2009). Nutrition and muscle protein synthesis: a descriptive review. The Journal of the Canadian Chiropractic Association, 53(3), 186–193. http://www.ncbi.nlm.nih.gov/pubmed/19714233
- Gibala, M. J. (2007). Protein metabolism and endurance exercise. Sports Medicine, 37(4–5), 337–340. https://doi.org/10.2165/00007256-200737040-00016
- Damas, F., Phillips, S. M., Libardi, C. A., Vechin, F. C., Lixandrão, M. E., Jannig, P. R., Costa, L. A. R., Bacurau, A. V., Snijders, T., Parise, G., Tricoli, V., Roschel, H., & Ugrinowitsch, C. (2016). Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. Journal of Physiology, 594(18), 5209–5222. https://doi.org/10.1113/JP272472
- Konopka, A. R., Castor, W. M., Wolff, C. A., Musci, R. V., Reid, J. J., Laurin, J. L., Valenti, Z. J., Hamilton, K. L., & Miller, B. F. (2017). Skeletal muscle mitochondrial protein synthesis and respiration in response to the energetic stress of an ultra-endurance race. Journal of Applied Physiology, 123(6), 1516–1524. https://doi.org/10.1152/japplphysiol.00457.2017
- Wilkinson, S. B., Phillips, S. M., Atherton, P. J., Patel, R., Yarasheski, K. E., Tarnopolsky, M. A., & Rennie, M. J. (2008). Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. Journal of Physiology, 586(15), 3701–3717. https://doi.org/10.1113/jphysiol.2008.153916
- Atherton, P. J., & Smith, K. (2012). Muscle protein synthesis in response to nutrition and exercise. In Journal of Physiology (Vol. 590, Issue 5, pp. 1049–1057). Wiley-Blackwell. https://doi.org/10.1113/jphysiol.2011.225003
- Bautmans, I., Van Puyvelde, K., & Mets, T. (2009). Sarcopenia and functional decline: Pathophysiology, prevention and therapy. In Acta Clinica Belgica (Vol. 64, Issue 4, pp. 303–316). Acta Clin Belg. https://doi.org/10.1179/acb.2009.048
- Chargé, S. B. P., & Rudnicki, M. A. (2004). Cellular and Molecular Regulation of Muscle Regeneration. In Physiological Reviews (Vol. 84, Issue 1, pp. 209–238). Physiol Rev. https://doi.org/10.1152/physrev.00019.2003
- Biolo, G., Tipton, K. D., Klein, S., & Wolfe, R. R. (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. American Journal of Physiology - Endocrinology and Metabolism, 273(1 36-1). https://doi.org/10.1152/ajpendo.1997.273.1.e122
- Biolo, G., Maggi, S. P., Williams, B. D., Tipton, K. D., & Wolfe, R. R. (1995). Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology - Endocrinology and Metabolism, 268(3 31-3). https://doi.org/10.1152/ajpendo.1995.268.3.e514